Shruthi Bandyadka
C U R R E N T . P R O J E C T S
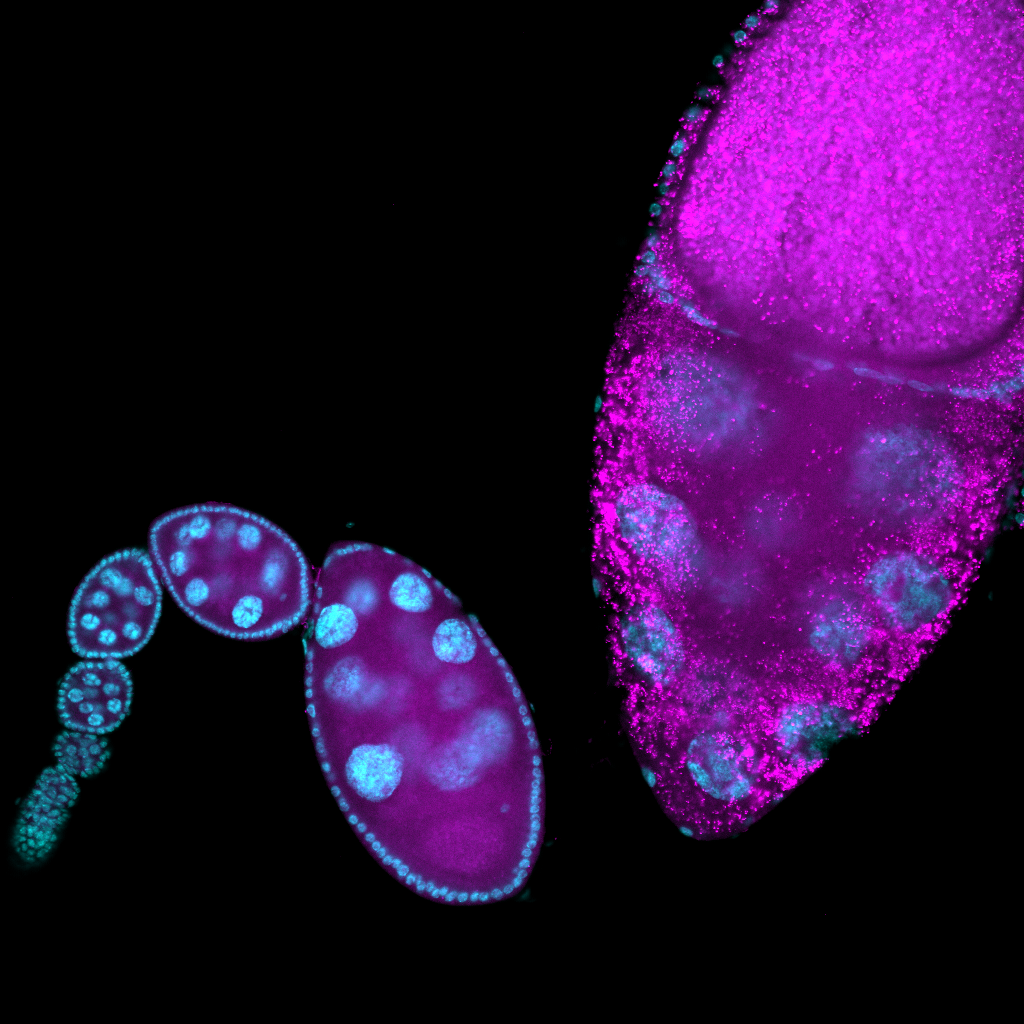
(1) Molecular differences between phagoptosis and apoptosis Drosophila melanogaster ovaries allow us to study multiple cell death and clearance paradigms. The epithelial follicle cell layer is able to acidify and kill the germline-derived nurse cells during late-oogenesis in a process known as phagoptosis. The same follicle cells also engulf nurse cells dying via apoptosis and autophagic pathways when the organism is deprived of protein-rich food sources. How are follicle cells able to undergo strikingly different morphological configurations and achieve nurse cell clearance by employing context-dependent biochemical pathways? I am investigating this problem by comparing transcriptional, translational, and proteomic profiles of epithelial follicle cells during phagoptosis and starvation-induced death to identify molecular candidates that uniquely regulate these distinct cell death regimes. (manuscript in prep)
(2) The role of Vacuolar-ATPases in phagoptosis V-ATPases are trans-membrane proteins that shuttle H+ ions typically between the lysosomal luminal and peripheral regions. During phagoptosis, V-ATPases are localized to the plasma membrane of follicle cells, facilitating the acidification of nurse cells. I am investigating the co-transcriptional mechanisms by which V-ATPases are trafficked and targeted to the plasma membrane using RNA-seq and RNA-FISH.
(3) Plasma membrane dynamics in phagoptosis Follicle cells undergo striking cytoskeletal remodeling to migrate and extend their processes into the nurse cell junctions. By perturbing the genetic candidates identified in (1) in vivo , I am quantifying and modeling the morphodynamics of follicle cells by using live-cell confocal imaging paired with a deep-learning model I am developing.